Log in to view meeting materials such as meeting slides and recordings
2025 Fall CTSA Program Annual Meeting

2025 Fall CTSA Program
Annual Meeting
ARLife: Building a Cross-Institutional, Cross-Sector, Real-World Data Platform for Lifespan Research in Arkansas
Hub not available
Abstract
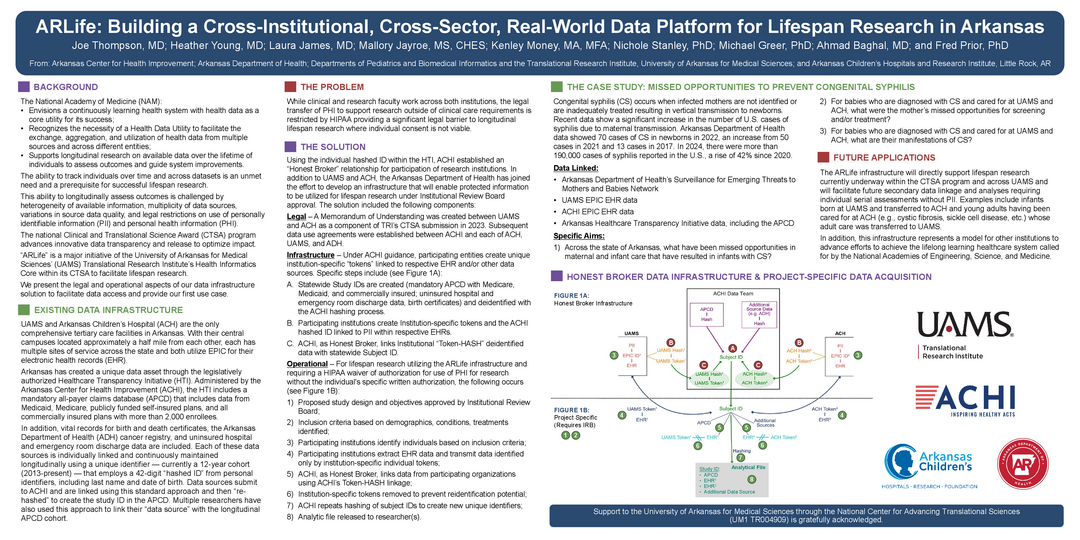
TITLE: ARLife: Building a Cross-Institutional, Cross-Sector, Real-World Data Platform for Lifespan Research in Arkansas
BACKGROUND: The National Academy of Medicine envisions a continuously learning health system with data as a core utility for success. Clinical and Translational Science Award (CTSA) programs have leveraged electronic health care data for COVID-19 research at the national level. However, individual state-based approaches for clinical research using electronic health records data, as well as data from public health departments and health insurance claims has received less attention. Barriers to compiling data from multiple health-related entities include entity-specific missions and regulations, data privacy and security, variations in data quality, and logistical challenges associated with patient name changes over time. The Translational Research Institute (TRI) at the University of Arkansas for Medical Sciences (UAMS) developed ARLife to overcome data linkage challenges and to support research initiatives in lifespan research. We present the legal and operational aspects of ARLife, TRI’s initiative to overcome these barriers in support of lifespan research.
AIMS/PUPOSE: To enable linked longitudinal data from multiple institutions without individually informed consent to be utilized by researchers for lifespan research.
METHODS: TRI leveraged existing relationships with representatives from two hospitals, the Arkansas Department of Health, and an independent health policy center to build a viable mechanism to create analytic files for lifespan research. We describe the institutional agreements, “honest broker” data infrastructure, and project-specific processes for data acquisition.
RESULTS: The method of combining existing data including a mandatory all-payer claims database, state health department data, EPIC electronic health record data from the two tertiary care centers serving Arkansas adults and children, UAMS and Arkansas Children’s Hospital, respectively, is demonstrated. A case study of initial application for congenital syphilis is provided.
CONCLUSION: TRI’s ARLife infrastructure directly supports lifespan research currently underway and represents a model for other institutions to advance efforts to establish longitudinal studies.
BACKGROUND: The National Academy of Medicine envisions a continuously learning health system with data as a core utility for success. Clinical and Translational Science Award (CTSA) programs have leveraged electronic health care data for COVID-19 research at the national level. However, individual state-based approaches for clinical research using electronic health records data, as well as data from public health departments and health insurance claims has received less attention. Barriers to compiling data from multiple health-related entities include entity-specific missions and regulations, data privacy and security, variations in data quality, and logistical challenges associated with patient name changes over time. The Translational Research Institute (TRI) at the University of Arkansas for Medical Sciences (UAMS) developed ARLife to overcome data linkage challenges and to support research initiatives in lifespan research. We present the legal and operational aspects of ARLife, TRI’s initiative to overcome these barriers in support of lifespan research.
AIMS/PUPOSE: To enable linked longitudinal data from multiple institutions without individually informed consent to be utilized by researchers for lifespan research.
METHODS: TRI leveraged existing relationships with representatives from two hospitals, the Arkansas Department of Health, and an independent health policy center to build a viable mechanism to create analytic files for lifespan research. We describe the institutional agreements, “honest broker” data infrastructure, and project-specific processes for data acquisition.
RESULTS: The method of combining existing data including a mandatory all-payer claims database, state health department data, EPIC electronic health record data from the two tertiary care centers serving Arkansas adults and children, UAMS and Arkansas Children’s Hospital, respectively, is demonstrated. A case study of initial application for congenital syphilis is provided.
CONCLUSION: TRI’s ARLife infrastructure directly supports lifespan research currently underway and represents a model for other institutions to advance efforts to establish longitudinal studies.
Authors
First Author
Joe Thompson, MD
Contributing Authors
Heather Young, MD
Laura James, MD
Mallory Jayroe, MS, CHES
Kenley Money, MA, MFA
Nichole Stanley, PhD
Michael Greer, PhD
Ahmad Baghal, MD
Fred Prior, PhD
Poster